Latest News
25. November 2011
The first presentation of product Ovosan in Russia at exhibition Apteka 2011. Come and see us.
5. November 2011
REDUCCIA s.r.o. seeks distributors of the original nutritional supplement OVOSAN for the Russian market.
Information for specialists
Development of the Preparation
Ovosan is an original Czech product containing biologically active substances that are characterised by the ability to effectively aid in the treatment and prevention of tumour diseases.
Systematic research of the main substance of this preparation began in the early 1980s. In the 1990s, it was followed with the development of industrial production and the first package of capsules appeared in 1998.
The preparation has been approved as a dietary supplement; however, its launch in the 'drug' form is moving forward. Researches and studies performed to date give this preparation a chance of playing a vital role in the treatment of tumour diseases as well as in the general reinforcement of immunity.
Indication and Recommended Dosage
Ovosan is provided both in support of prevention and as a supportive preparation for the diseases noted below. The table offers examples of administration schemes for individual indications. It is possible to adapt the dosage individually; however, arriving at the desirable effects necessitates the taking of the recommended minimum amount of capsules in one treatment cure.
Mechanism of action 1)
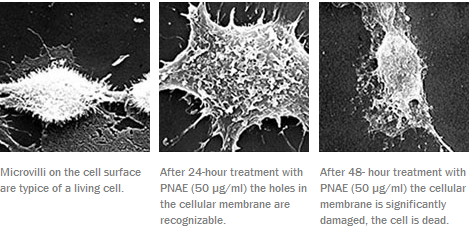
The active substance is ether fosfolipid PNAE (plasmanyl-N-acyl-etanolamin). Its activity is based ona different metabolism of ether phospholipids in healthy and tumour cells. Healthy cells carry the enzyme alkylglycerolmonooxygenase that cleaves an ethereal bond in the PNAE molecule. The resulting fragments are further used for biosynthesis of lipids and phospholipids that form an essential part of cell membranes. This enzyme is absent or almost inactive in tumour cells which leads to the accumulation of ether phospholipides PNAE in the tumour cell membranes and their destruction, while healthy cells remain undamaged.
Another positive quality is the inhibitory effect on protein kinase C (PKC) that appears in tumour cells in elevated concentration. PKC is the internal messenger of signal in the cell. It affects enzymatic systems that play an important role in regulating cell proliferation. PKC is also inhibited by other substances with anti-tumour effect such as tamoxifen 2), adriamicin 3), calphostin C 4), that are used for the clinic treatment of cancer. In contrast to these cytostatic drugs, the competitive inhibition of PKC by the semi-synthetic PNAE preparation has no toxic effects. 5)
Furthermore, an empiric finding exists indicating that Ovosan administration supports functions of the immunity system. It was successfully applied, for example, in the treatment of auto-immune and allergic diseases.
Microphotograph of the HEp-2 human tumour cell in the tissue culture:
References:
1) KÁRA J.: Ether Phospholipid PNAE against tumour cells. Prevention and therapy of metastases, 2001.
2) O´BRIEN C.A., LISKAMP R.M., SOLOMON D.H., WEINSTEIN I.B.: Inhibition of protein kinase C by Tamoxifen. Cancer res., 45, 2462-2465, 1985.
3) ZHAO F.K., CHUANG L.F., ISRAEL M., CHUANGR.Y.: Adriamycin interacts with diacylglycerol to inhibit human leukaemia protein kinase C. Anticancer Research, 9, 225-230, 1989.
4) KOBAYASHI E., NAKANO H., MORIMOTO M., TAMAOKI T.: Calphostin C (UCN - 1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem. Biophys.Res.Commun., 159, 548-553, 1989.
5) KÁRA J.: Ether-fosfolipidy v onkologii, Chemické listy 87, 58-63, 1993.
Shortened information
Name of the preparation: Ovosan
Composition: In each capsule with 500 mg content there are: 150 mg of egg extract (specific egg phospholipids PNAE), 350 mg of edible sunflower oil, maximum 1mg of cholesterol, the rest are edible gelatine, glycerol and water. The energetic value of the gelatine capsule is 23 kJ (6 kcal). Precipitate, if any, is not a defect.
Indication: Supportive preparation in tumour diseases. Reinforcement of stamina. Favourable effect on the psychic and physical activity, faster regeneration. Protection of healthy, undamaged cells of the body and harmonisation of the energetic balance of organism.
Contraindication: Contraindications are not known, the preparation does not contain either preserving agents or synthetic dyes and it is not addictive. A long-term observation proved its non-toxicity for healthy cells.
Notice: The preparation is not intended for children under the age of 3 years. Put out of reach of children. It does not substitute a varied diet.
Package: 90 capsules with 500 mg content.
Dosage: Recommended daily dosage is 2 capsules, in states of exhaustion and serious impairment of organism up to 9 capsules daily. Take the capsules after meal.
Special notice: For a better elimination of toxic substances it is suitable to ensure a higher intake of liquids, approximately 2.5 to 3 litres per day. During this period a short-term subjective occurrence of a bigger fatigue, irritability, headaches or somnipathy can be witnessed as a reaction to the eliminated toxic substances. A long-term administration (6-8 weeks) results in the reinforcement of stamina. The exceeding of the stated doses should be consulted with the doctor.
Decision No.: HEM-350-25.4.01/11472. PN - 141178. (pdf for downloads) Czech Republic
Decision holder: AREKO, spol. s r.o., Dobronická 635, 148 25 Praha 4, Czech Republic. Tel. +420 261 112 872, e-mail: info@areko.eu
Storage: Keep in a dry place with the temperature up to 25 °C.
Expert publication
Extracts from the professional publication written by RNDr. Jindřich Kára DrSc. "Ether fosfolipid PNAE against the tumour cells: Prevention and therapy of metastases", published by AREKO in May 2000 on the occasion of the international oncological symposium held on 5 and 6 May 2000 in Prague - Pruhonice under the auspices of "Liga proti rakovine" Prague.